stimOS for …
(choose your profession)
supplier/industry
Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod
patients
Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod
stimOS GmbH is …
By using the
latest technologies
and applying strict quality
control procedures, we’ve
become known for having
the best products in
the industry.
Introducing
Mimicking Bone Technology (MBT)
There are too many surface treatments on the market using different technologies and adressing various needs: The idea of stimOS‘ competitors is, to simply apply a coating on the implant surface. But solutions like this are problematic.

The Problem
The coating process often damages the implant material and there are serious problems with regard to abrasion and delamination and/or metal ion release.
There is ONE SOLUTION:
Based on one biochemical principle, using one technology, addressing ALL important market needs.
With MBT, stimOS offers the surgeon and his patients a completely new solution. Rather than relying on coating technologies, the company restructures the implant materials biochemically by means of a covalently bound activation layer. Doing this stimOS gives inert materials biological features close to nature.
With MBT the company created an evolutionary material with unique characteristics made to support early bone formation and proper anchorage that can be described as cell-attractive and anti-inflammatory. MBT is one technology concept addressing the most important needs of surgeons and patients demanding for high-performance implant materials to be used in spinal, dental, CMF and other orthopedic applications.
01
MBT osseo
For all kind of implants that need osseointegration. Patent protected.
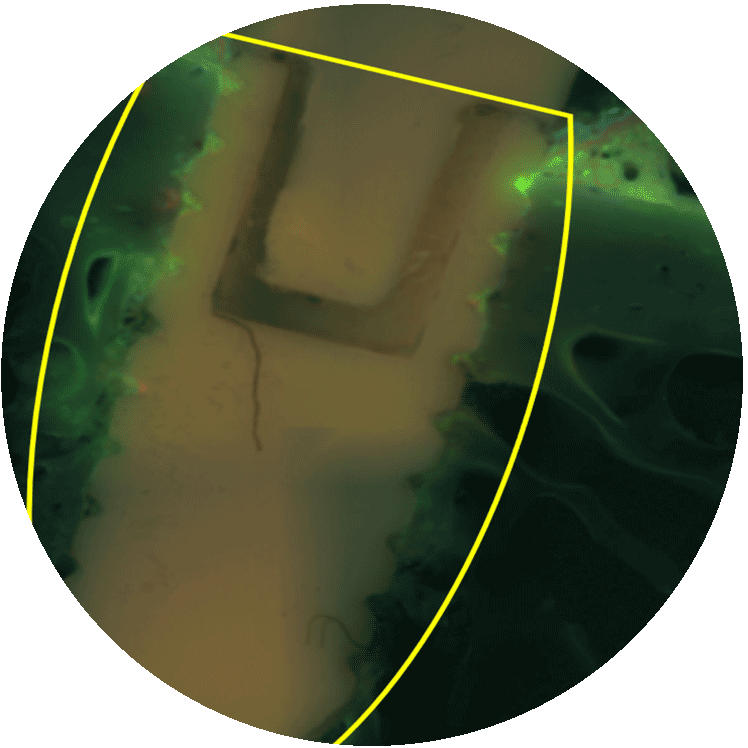
02
MBT biocide

Bio-inspired, non-toxic, antibacterial implant coating. Patent protected
03
MBT active
Providing stable anchorage for dental PEEK implants. Patent protected.
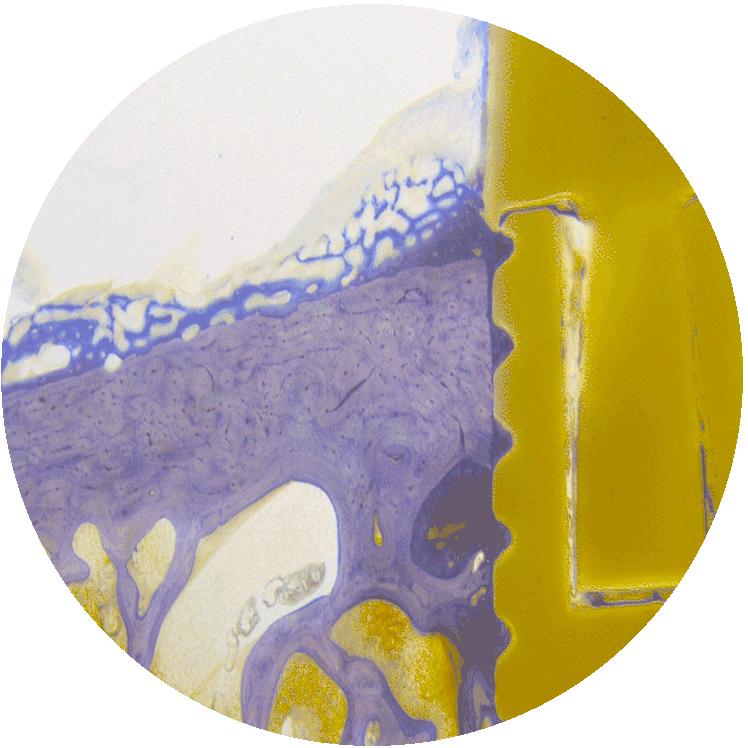
04
MBT dental

Bio-inspired, non-toxic, antibacterial implant coating. Patent protected
05
MBT protect
Drug delivery directly to the intended part of the human body. Patent pending
Read more about Mimicking Bone Technology here.
01
MBT osseo

For all kind of implants that need osseointegration. Patent protected.
02
MBT biocide

Bio-inspired, non-toxic, antibacterial implant coating. Patent protected.
03
MBT active

Providing stable anchorage for dental PEEK implants. Patent protected.

Bio-inspired, non-toxic, antibacterial implant coating. Patent protected.
04
MBT dental
05
MBT protect

Drug delivery directly to the intended part of the human body. Patent pending.
Read more about Mimicking Bone Technology here.
Mimicking Bone Technology:
Stealth Technology for implants
To give the patient best treatment possible and to ensure a life free of pain post-operatively stimOS GmbH has developed a unique biochemical procedure that initiates early and healthy bone formation, gives best anchorage also in osteoporotic bone, and can be described as anti-inflammatory.
The company’s mission is to transform implant surfaces from an artificial barrier in the patient´s body into a bone-identic implant body interface to avoid inflammatory reactions and re-operations.
„We do
this because
conventional implant
materials are not really
made to support bone
healing, especially not
in the case of revisions
and with patients with
osteoporotic
bone.“
Dietmar Schaffarczyk,
CEO of stimOS GmbH.
Implants that do not heal in the patient’s body often need to be replaced. These additional procedures are not uncommon and additionally burden the health of the patient, which is already weak: German Society for Implantology reports about 160.000 dental implants that must be replaced per year as they do not heal properly. German AOK states that 40% of all spine surgeries end in a revision. According to the German federal bureau of statistics, more than 16.000 artificial hips and 26.000 artificial knees per year must be re-operated and replaced because the implants do not anchor or heal in properly. This is where the development of stimOS researchers takes place: MBT will be crucial to avoid revisions due to failed operations.
The problem of implant loosening and inflammatory reactions due to inert implant materials is known already for a long time: Until now this problem was not addressed successfully.
Publication Download Center
Fact sheets and certificates
Transparency Strategy
stimOS takes on new challenges for the benefit of patient safety, and aiming for new standards and benchmarks
ISO 13485:2016 certificate
stimOS will market it‘s own unique implant portfolio and will licence MBT for legal manufactures, that are interested in MBT technology to enhance the performance of their own hip, knee, spinal or CMF implants.
Press review
December–January 2021
stimOS MBT booklet
Platzhaltertext
stimOS spineFuse booklet
Platzhaltertext
Whitepapers & Scientific Publications
Following our Transparency Strategy, all articles, scientific papers, brochures and technical documentations are published „open source“.
Critical evaluation of different coating technologies
Technology Verification File
Animal Model I
Technology Verification File
Animal Model II
Technology Verification File
KPI Analysis of stimOS MBT Technology
Case Study about stimOS spineFuse implants
The Need for a Quality Standard for Coatings
Scientific Paper analyzing the quality standard of different coatings
S.P.E.L. Quality Evidence Level Evaluation of Surface Functionalization Technologies
Scientific Paper describing how to proof Safety and Performance of Surface Functionalizations
S.P.E.L. Safety and Performance Evidence Level for 3D printed Medical Devices
Scientific Paper and Perspective describing S.P.E.L for Additive Manufacturing
On the Future Design of Dental Implants
Scientific Paper and Review describing Possibilities in Dental Implant Improvement
New Benchmark in Surface Functionalization Technologies
Industry Article describing stimOS surface Technologies as Future Benchmark Technologies
Publication Download Center
Fact sheets and certificates
Transparency Strategy
stimOS takes on new challenges for the benefit of patient safety, and aiming for new standards and benchmarks
ISO 13485:2016 certificate
stimOS will market it‘s own unique implant portfolio and will licence MBT for legal manufactures, that are interested in MBT technology to enhance the performance of their own hip, knee, spinal or CMF implants.
Press review
December–January 2021
stimOS MBT booklet
Platzhaltertext
stimOS spineFuse booklet
Platzhaltertext
Whitepapers & Scientific Publications
Following our Transparency Strategy, all articles, scientific papers, brochures and technical documentations are published „open source“.
Critical evaluation of different coating technologies
Technology Verification File
Animal Model I
Technology Verification File
Animal Model II
Technology Verification File
KPI Analysis of stimOS MBT Technology
Case Study about stimOS spineFuse implants
The Need for a Quality Standard for Coatings
Scientific Paper analyzing the quality standard of different coatings
S.P.E.L. Quality Evidence Level Evaluation of Surface Functionalization Technologies
Scientific Paper describing how to proof Safety and Performance of Surface Functionalizations
S.P.E.L. Safety and Performance Evidence Level for 3D printed Medical Devices
Scientific Paper and Perspective describing S.P.E.L for Additive Manufacturing
On the Future Design of Dental Implants
Scientific Paper and Review describing Possibilities in Dental Implant Improvement
New Benchmark in Surface Functionalization Technologies
Industry Article describing stimOS surface Technologies as Future Benchmark Technologies